A leading probiotic nutritional supplement company approached us to participate in a viability comparison study. They provided two probiotic strains for us to process and optimize for maximum shelf stability, which would be compared against the same strains processed by a competitor’s freeze-drying service.
Case Study
LyoLock™ Process Improves Nutritional Supplement Probiotic Viability

Study details
Our goal and strategy
Confident in our LyoLock process and expertise in microbial stabilization, we aimed to retain higher levels of time-zero viability through an accelerated shelf-life study.
We collaborated closely to define a comprehensive testing plan covering all critical process steps to ensure product stability.
Approach
Our Technical Services team collaborated with theirs to develop a plan tailored to their specific inputs and product treatments to achieve optimal retention of probiotic viability.
We integrated specialized process and packaging solutions, considering factors such as fermentation, concentration methods, and their proprietary cryopreservative formulation.
Outcome
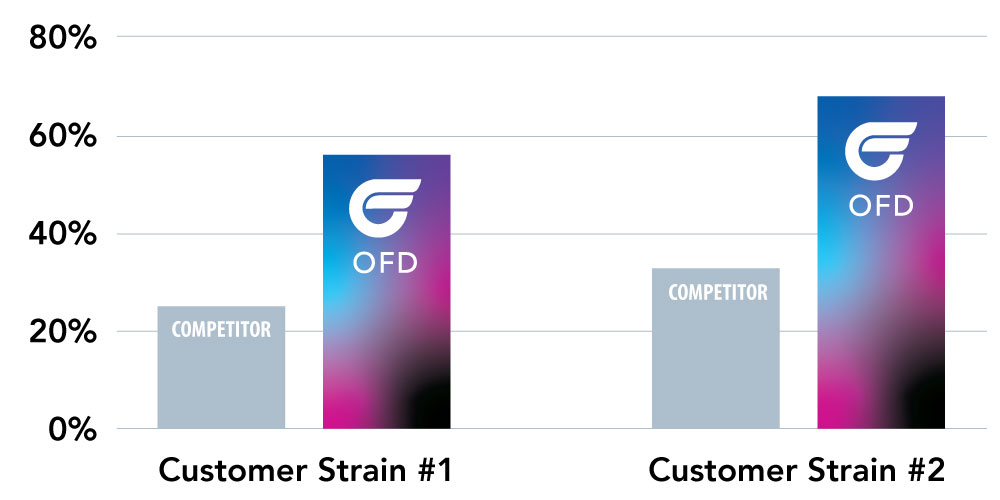
The results indicate that we successfully applied our LyoLock process to achieve a higher relative percentage survival rate for both probiotic strains versus the competitive freeze-drying option.
As a result of this study, OFD Life Sciences won the business and continues to collaborate with and support this leading probiotic nutritional supplement company in the marketplace.

Expert Insights and Proven Strategies to Empower Your Growth
Check out our curated educational and informational resources to stay current with advances in lyophilization technology and uses.
Quick Links
Get In Touch
525 25th Avenue SW
Albany, OR 97322
customerservice@ofd.com
541.926.6001

