What Is a Lyophilization Cycle?
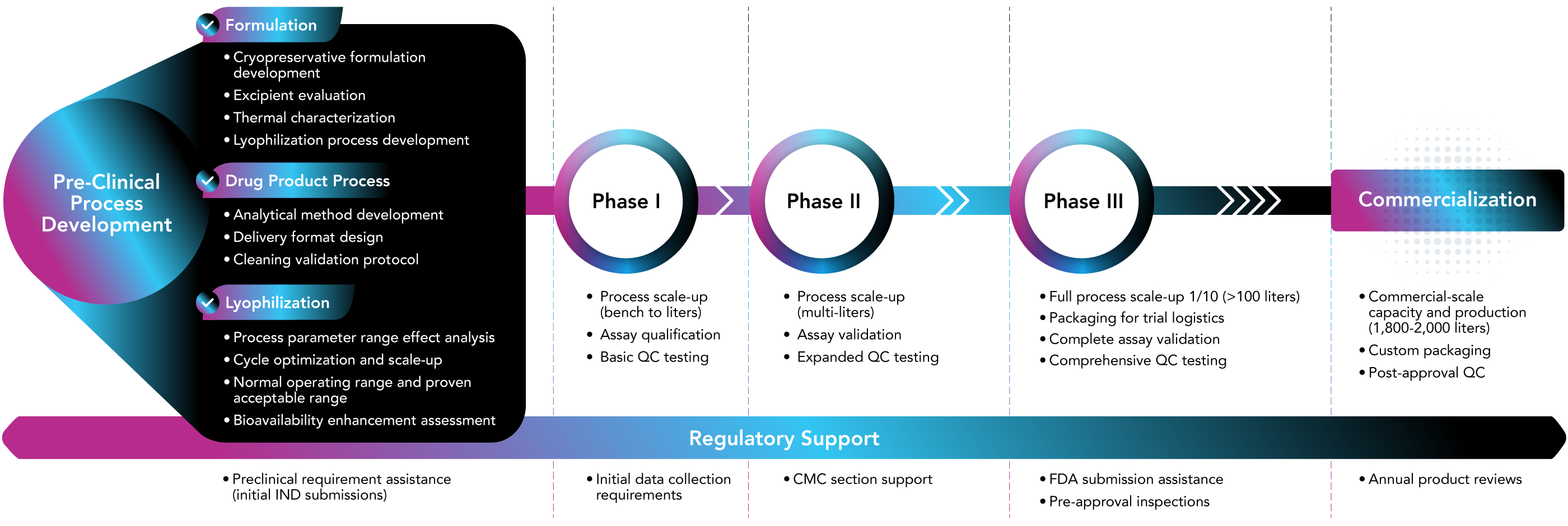
Lyophilization (or freeze drying) is an indispensable technology that can stabilize sensitive formulations during the final stages of pharmaceutical production. While many are familiar with lyophilization in vials for injectable products, bulk lyophilization of formulated API can provide many benefits that improve druggability and provide a wider range of delivery options. In both cases, the process involves a carefully controlled sequence of uniform depth of fill, freezing, and drying steps, where time, temperature, and pressure must be precisely managed. That sequence is known as the lyophilization cycle and it can determine whether a product remains stable, potent, and ready for use.
Each product requires a cycle that matches its unique formulation, delivery format, and sensitivity to stress. Developing that cycle is one of the most important steps in creating a successfully lyophilized product and it’s where much of the real work happens behind the scenes.
Why Cycle Development Matters
Each of the lyophilization cycle’s freezing, primary drying, and secondary drying phases serves a distinct purpose. Small adjustments to temperature, pressure, or timing can have a major impact on product quality, efficiency, and stability. These small adjustments are predicated upon heat and mass transfer capacities and control of the lyophilization equipment.
A well-designed lyophilization cycle directly impacts how the product performs, how efficiently it can be manufactured, and how easily it meets regulatory expectations. Developing that cycle starts with understanding the unique demands of the product, the process, and the equipment.
Tools and Techniques for Optimizing Cycles
A variety of tools and techniques are used to develop and refine lyophilization cycles. The table below outlines key examples, their roles in optimization, and whether they are exclusive to OFD Life Sciences.
| Tool or Technique | Type | How It Optimizes the Cycle |
|---|---|---|
| Differential Scanning Calorimetry (DSC) | Analytical Tool | Identifies key thermal transitions like glass transition and collapse temperatures to define safe processing limits. |
| Freeze-Drying Microscopy (FDM) | Analytical Tool | Visualizes structural changes during drying to determine the point of collapse and guide primary drying conditions. |
| Pirani vs. Capacitance Manometer Comparison | Cycle Monitoring Technique | Compares pressure sensor readings to confirm when the sublimation and desorption rates have reduced to levels that indicate the end of drying for a given set of parameters. |
| LyoLock™ System | Integrated Control System (Exclusive to OFD) | Applies precise control strategies across development and manufacturing to protect product quality, stability, and bioavailability. |
| OFD Technical Services | Service Offering (Exclusive to OFD) | Provides tailored support from early formulation to scale-up, ensuring the cycle is matched to the product and process needs. |
Scalability and Technology Transfer
A robust lyophilization cycle is necessary to scale up and transfer to a larger capacity lyophilizer. Small-scale development work must translate reliably into clinical and full-scale production, starting with a cycle designed to hold up under changing conditions. The more consistent the thermal and pressure profiles, the easier it translates to reproducing results at different volumes and batch sizes.
At OFD Life Sciences, scale-up and tech transfer are built into the cycle development process from the start. The same team that designs the initial cycle also supports its optimization, validation, and integration into production-scale equipment. Because OFD designs and builds its own lyophilization systems, cycle parameters developed at lab scale are directly transferable to Clinical and Commercial operations. That continuity helps reduce variability, improve success rates, and shorten the path to market.
Ultimately, cycle development is about setting it up for long-term success, preserving quality, supporting scale, and protecting every dose from development through distribution. We design every lyophilization cycle with your success in mind.
Want to make sure your cycle delivers consistent results at any scale? Let’s talk.